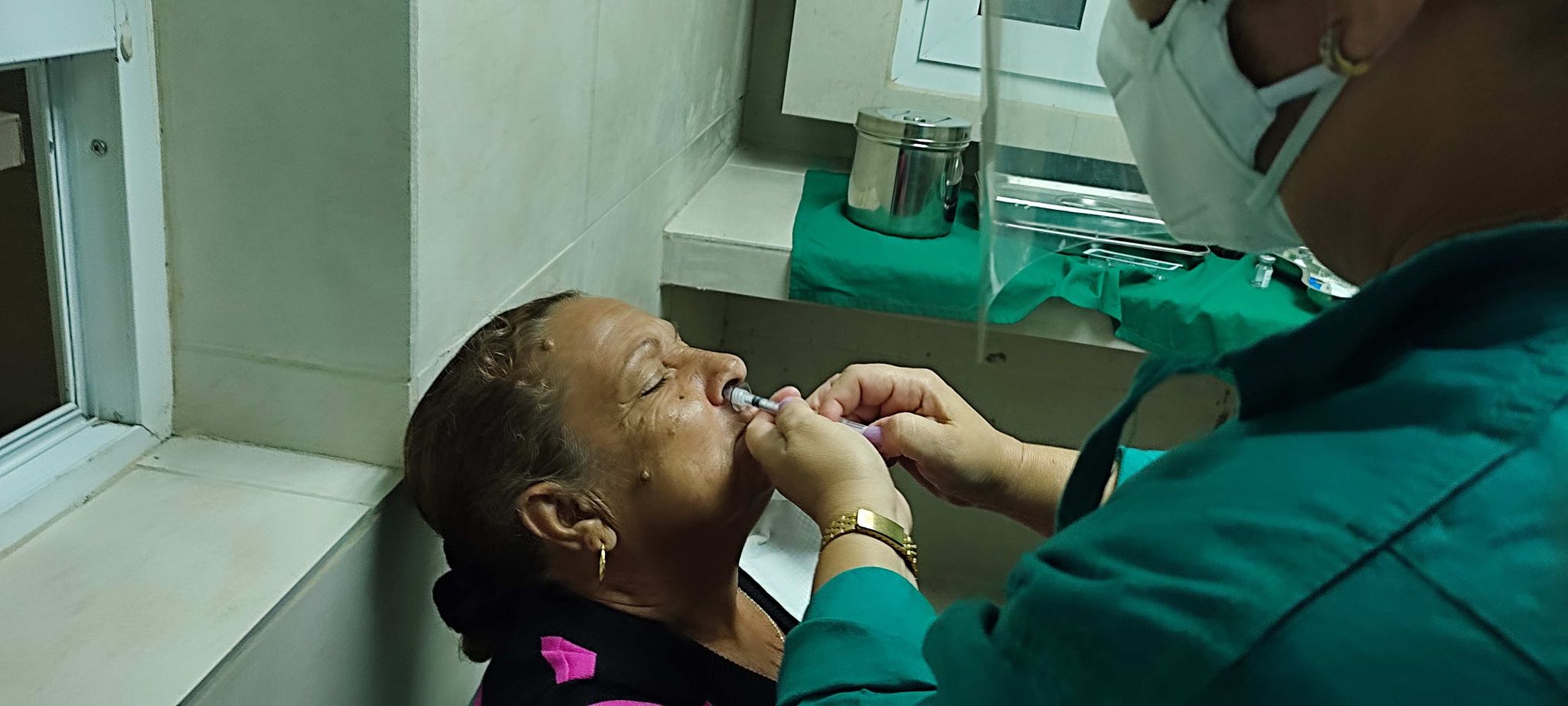
CAMAGUEY, CUBA.- This province is included as of today in the Phase II clinical trial with the vaccine candidate Mambisa and the Abdala vaccine in COVID-19 convalescents, carried out at the Hermanos Ameijeiras Surgical Clinical Hospital in Havana.
The study is expected to benefit about 200 people, between 19 and 80 years old, who must have been discharged from the hospital for two months or more and must not have received any dose of any anti-COVID-19 immunogen, Ailyn Nordelo Valdivia, head of the research and development department of the Center for Genetic Engineering and Biotechnology (CIGB) of the territory, told the Cuban National Agency.
They can be allergic or not to thiomersal and, if they suffer from a chronic disease, such as diabetes mellitus and arterial hypertension, this must be compensated at the time of vaccination, she added.
As this is a clinical trial, there is a control group that would receive a dose of Abdala, but without thiomersal, by intramuscular injection, as there is randomization and this immunogen can be administered, or Mambisa by nasal application.