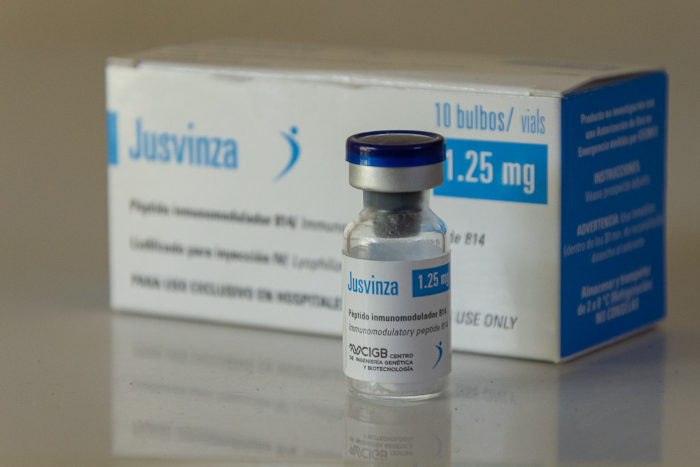
CAMAGÜEY- More than 96% of effectiveness has the use of the Jusvinza (CIGB 258) in the Red Zones of the territory. Its application is due to the participation of the province in the study protocol of this drug in the confrontation with COVID-19.
Jusvinza, an immunomodulatory synthetic peptide 814, was developed by the Center for Genetic Engineering and Biotechnology 6GB and after a rigorous evaluation of the file presented by the group of biotechnology and pharmaceutical industries BioCuba Farama, it received authorization on June 17, 2020 for emergency use against SARS-CoV-2.
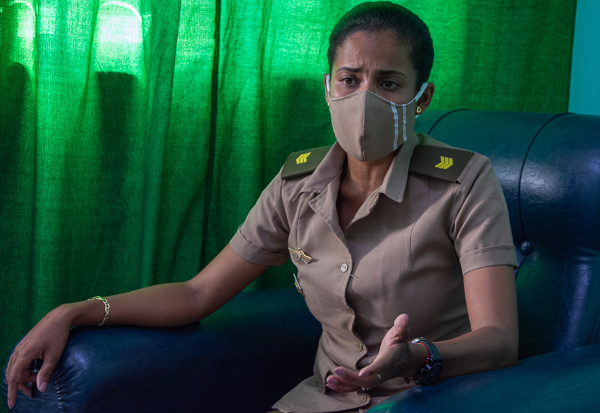 "The first experience with the drug here," said Captain Zoe Roca Nuñez, research coordinator and specialist in Comprehensive General Medicine and in intensive and emergency medicine in adults, was in September with very good results, especially in these serious and critical patients. In moderate and high risk patients it is much more efficient.
"The first experience with the drug here," said Captain Zoe Roca Nuñez, research coordinator and specialist in Comprehensive General Medicine and in intensive and emergency medicine in adults, was in September with very good results, especially in these serious and critical patients. In moderate and high risk patients it is much more efficient.
“Its function is to participate in the inflammatory stage, the so-called cytokine storm acts as an anti-inflammatory with another advantage, it also acts on the patient's immunity, at the same time it participates in the high inflammatory part and helps to raise the patient's defenses. At the beginning, when only its use was in serious cases, we did not have many cases because there have not been many cases that have reached these complications in our hospitals ”.
The 31-year-old, who has already rotated through the Red Zone six times, assured that “its application in an early stage of the disease has left as clinical and radiological evidence a more expeditious evolution to the recovery of the sick. Its application in children is still being evaluated ”.
Jusvinza is a synthetic immunomodulatory peptide derived from the cellular stress protein HSP60. Preclinical studies have shown decreased levels of pro-inflammatory cytokines.
Initially it was indicated for the treatment of severe and critical patients, confirmed by SARS-CoV2, adults, hospitalized in intensive care units with confirmed multifocal interstitial pneumonia or signs and symptoms that indicate the need for oxygen therapy to maintain SO2> 93%, with worsening of lung function, as well as with suspicion or identification of a state of hyperinflammation.
This product, according to ACN, was designed for the treatment of diseases such as rheumatoid arthritis, but due to its abilities to reduce the hyperinflammation caused by SARS-CoV-2 in serious, critical patients and avoid complications in high-risk patients, it is authorized its use against the new coronavirus. José Ángel Portal Miranda, Minister of Public Health, assured that it is the first innovative drug in the country based on peptide synthesis.
Translated by Linet Acuña Quilez