This Wednesday, 48 hours after receiving the first dose of Abdala, the boys returned to the vaccinations. In the East polyclinic, in the city of Camagüey, "Nurse" Baby, the same nurse who injected them on Monday, measured their temperature, their blood pressure and asked them a lot of questions about how they felt that day after immunization. They are adolescents between 12 and 18 years old and participate in phase II of the Ismaelillo study. On Thursday they will check them again. This is how rigorous the process of the clinical trial with the Cuban vaccine Abdala is, which evaluates its safety and immunogenicity in the pediatric population.
 On Monday, July 26, children and relatives participating in the Ismaelillo study thanked the Revolution and especially the Cuban scientists for their efforts, along with the Health personnel, for protecting the people against COVID-19. The two phases of the first stage have included volunteers from 12 to 18 years old, and then it will reach the little ones from 3 to 11.
On Monday, July 26, children and relatives participating in the Ismaelillo study thanked the Revolution and especially the Cuban scientists for their efforts, along with the Health personnel, for protecting the people against COVID-19. The two phases of the first stage have included volunteers from 12 to 18 years old, and then it will reach the little ones from 3 to 11.
 The adolescents and their families received a comprehensive explanation about the Abdala vaccine, the development of the clinical trial and the characteristics of phase II, which will measure their immunogenicity. Some fifteen days before, in two polyclinics in the city of Camagüey, the phase I group was vaccinated, which evaluated the safety.
The adolescents and their families received a comprehensive explanation about the Abdala vaccine, the development of the clinical trial and the characteristics of phase II, which will measure their immunogenicity. Some fifteen days before, in two polyclinics in the city of Camagüey, the phase I group was vaccinated, which evaluated the safety.
 Ismaelillo is a randomized, sequential phase I / II study without placebo. It is supported by compliance with good clinical practices and the established ethical and methodological rigor, with the participation of an independent data monitoring committee, made up of highly qualified professionals from outside the research sites and the Center for Genetic Engineering and Biotechnology, developer of the vaccine Abdala.
Ismaelillo is a randomized, sequential phase I / II study without placebo. It is supported by compliance with good clinical practices and the established ethical and methodological rigor, with the participation of an independent data monitoring committee, made up of highly qualified professionals from outside the research sites and the Center for Genetic Engineering and Biotechnology, developer of the vaccine Abdala.
 Family members and also adolescents sign their consent to participate in the study in an informed consent and receive the information that they can abandon it if they wish at any time.
Family members and also adolescents sign their consent to participate in the study in an informed consent and receive the information that they can abandon it if they wish at any time.
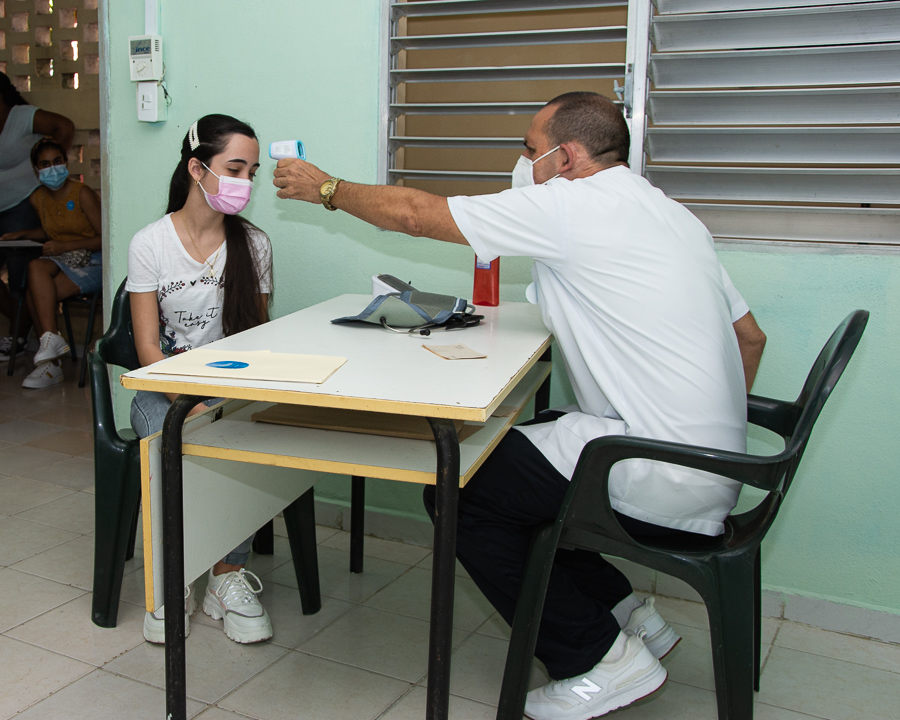 05 ismaelillo ensayo abdala
05 ismaelillo ensayo abdala
 A rigorous physical and clinical examination certifies that healthy children participate in the trial.
A rigorous physical and clinical examination certifies that healthy children participate in the trial.
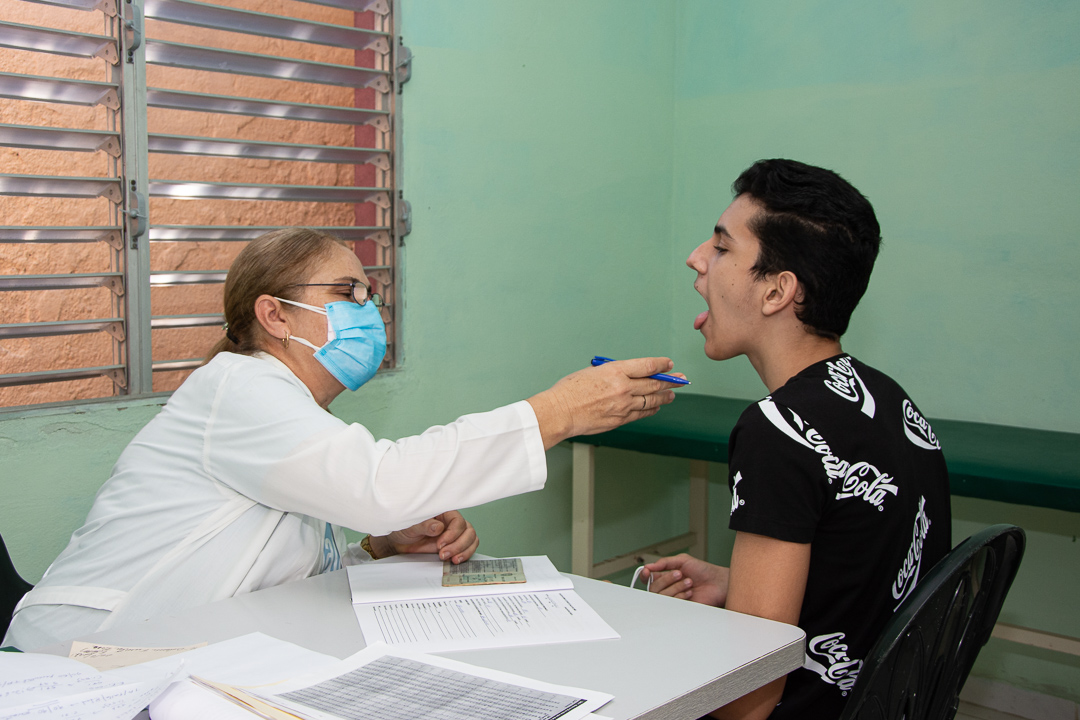 The pre-vaccination evaluation includes a Pediatric consultation that explores the personal and family health history and completes the physical examination.
The pre-vaccination evaluation includes a Pediatric consultation that explores the personal and family health history and completes the physical examination.
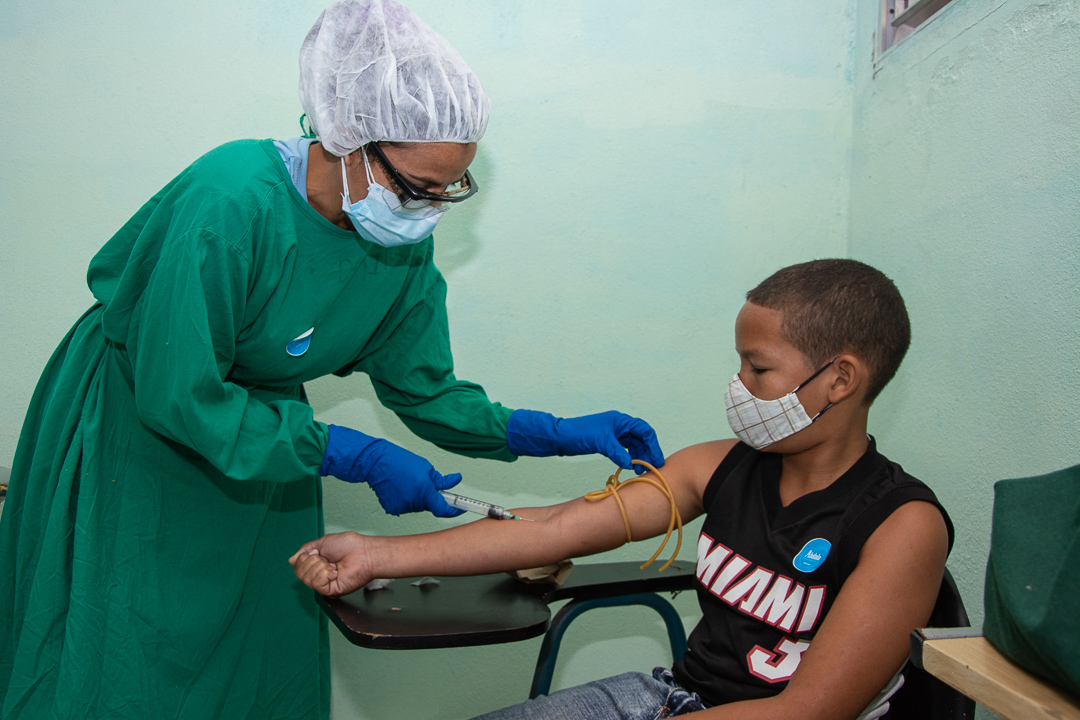 Blood tests measure, in addition to other parameters, the levels of antibodies present before vaccination. The sanple to compare will be repeated 28 days after the third dose is applied.
Blood tests measure, in addition to other parameters, the levels of antibodies present before vaccination. The sanple to compare will be repeated 28 days after the third dose is applied.
 Finally, the puncture! The immunization schedule foresees three doses intramuscularly in the arm, every 14 days, administered in the interval of zero, 14 and 28 days.
Finally, the puncture! The immunization schedule foresees three doses intramuscularly in the arm, every 14 days, administered in the interval of zero, 14 and 28 days.
Translated by Linet Acuña Quilez

